Study shows extreme contrast in ozone losses at North, South Poles

A new study shows just how dramatic the ozone loss in the Antarctic has been over the past 20 years compared to the same phenomenon in the Arctic.
The study found "massive" and "widespread" localized ozone depletion in the heart of Antartica's ozone hole region, beginning in the late 1970s, but becoming more pronounced in the 1980s and 90s.
The US government scientists who conducted the study said that there was an almost complete absence of ozone in certain atmospheric air samples taken after 1980, compared to earlier decades. In contrast, the ozone losses in the Arctic were sporadic, and even the greatest losses did not begin to approach the regular losses in the northern hemisphere, the researchers said.
"Typically the Arctic loss is dramatically less than the Antarctic loss," said Robert Portmann, an atmospheric scientist with the National Oceanic and Atmospheric Administration in Boulder, Colorado.
Scientists have been tracking the expanding ozone hole over Antarctica for some 20 years now.
In October, NASA scientists reported that this year's hole is the biggest ever, stretching over nearly 11 million square miles.
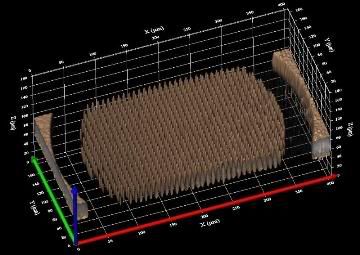
In Antarctica, local ozone depletion at some altitudes frequently exceeded 90 percent, and often reached up to 99 percent during the Antarctic winter in the period after 1980 compared to earlier decades, the researchers said.
In the Arctic, the losses occasionally peaked at 70 percent, and some losses of 50 percent were seen in the mid 1990s, when temperatures were particularly low, but the scale and scope of the problem was much less than what was seen in the Northern Hemisphere.
Recent studies have also pointed to large ozone losses in the Southern Hemisphere, but the NOAA researchers said their study showed that these events were rare and did not appear to signal a trend.
"We saw small to moderate ozone losses in the very coldest winters, when the stratospheric conditions are ripe for ozone loss, but they were rarer than we expected," said Portmann.
The study, published in the Proceedings of the National Academy of Sciences, was based on more than 40 years' of ozone readings from polar observation stations and balloon-borne measuring mechanisms.



 The findings, reported in the journal Science, come from the first analysis of dust fragments from Comet Wild-2, captured by NASA's Stardust spacecraft and brought to Earth in January 2006. Because comets are among the oldest objects in the Solar System, the team, which includes researchers from Imperial College London and the Natural History Museum, believes their sample of dust can provide insights into how Earth and other planets came to be formed.
The findings, reported in the journal Science, come from the first analysis of dust fragments from Comet Wild-2, captured by NASA's Stardust spacecraft and brought to Earth in January 2006. Because comets are among the oldest objects in the Solar System, the team, which includes researchers from Imperial College London and the Natural History Museum, believes their sample of dust can provide insights into how Earth and other planets came to be formed.